Introduction to Environmental Science
Notes from “Everyone’s World”, a 4-hour introductory course on Udemy.com taught by Dr. David Hackett, Retired Professor / Faculty of Arts and Science – Biology and Chemistry, formerly of Nipissing University in Ontario Canada. I thank Dr. Hackett for his review of and feedback on this article, which he has graciously provided. Illustrations by Escuela Biodiversidad, Costa Rica.
Why Study the Environment
How humanity interacts with the environment
The study of our environment starts with first acknowledging its crucial importance to our daily lives. Air, water, food, and natural energy - essential resources that we humans use - come from “the environment”, and our livelihoods depend on them. Indeed, Earth has been an ideal place for us: drinking water, clean air, and safe food, all satisfy our needs and enable us to lead healthy lives.
In addition, we also have wants, such as natural beauty, and biodiversity, which bring life satisfaction beyond our mere subsistence. Yet at the same time, our actions increasingly impact the environment, and often quite negatively.
It is therefore essential that we study environmental science: to ensure we can accomplish our goals, and to prevent others from accidentally (or malevolently) damaging it.
Environmental Science 101
Environmental science is an interdisciplinary field that brings together biology, chemistry, physics, geography, and ecology. And while that combination sounds interesting to some, communication on these issues is also key: the public needs to be involved, because humans regularly make decisions that affect the environment.
These could be large-scale, policy-based measures or small-scale, individual behaviors, or both. A key tenet to recognize at the outset is that it is our environment, and with that to challenge old myths that are inaccurate and dangerous.
Notions like “the solution to pollution is dilution”, or “Earth’s resources are limitless” are simply not true; many toxic chemicals dissolve in fat or other substances, but not in water and accumulate in bodies and organisms (for example, the metal mercury).
Mineral resources are finite, and non-renewable, and even large bodies of water have important equilibriums that can be disturbed if their chemical composition changes, thereby affecting fish populations.
These are just a couple of instances where common misconceptions can lead to disastrous, long-term effects of misunderstanding our world and the effect that human actions can have on it.
Fundamentals
Several components and concepts form the foundation of environmental science.
These are the core living elements of our world, such as plants, and animals, their habitats such as oceans, or the atmosphere, as well as a set of natural laws that govern their states and interactions with one another.
Let’s review them one by one:
The role of plants and trees in our world
1. Plants and Trees
Plants use solar energy to create biomass. They are habitat and food for other creatures, such as insects and bacteria. Insects are small, numerous, and play important roles: they eat plants, keeping them in check; they provide animal protein to other animals; some may pollinate plants and some break down waste.
Trees are important because they provide habitat, food and cover; they provide soil protection from rain and sun, they influence water quality and climate; they absorb carbon dioxide (CO2) from the atmosphere and generate oxygen (O2); they also provide jobs, products and are a significant part of the economy.
There are two main categories of trees:
Evergreen, softwood, or coniferous trees are found in cold climates because of their needle-like leaves that shed snow; and
Deciduous, hardwood trees, or broadleaf trees, found in warmer climates, that shed their leaves every year.
2. Animals: The Competitive Exclusion Principle
No two species can share the same niche in the same habitat for long. They must separate spacially or specialize, to avoid competition.
For example, the lynx in northern North America and the bobcat in the south of North America. The flying squirrel forages at night, whereas the red squirrel during the day. One is nocturnal, the other one is diurnal.
More organisms are born than can find a niche-space, so animals must specialize and subdivide their habitat to avoid competition.
More species and biodiversity can be supported. Competition can be between species (i.e. interspecies: see lynx and bobcat above) or intraspecies (i.e. two wolf packs fighting for the same territory)– and this is where natural selection leads to what’s often known as survival of the fittest: the slow deer gets eaten and cannot reproduce.
Faster deer get away and can reproduce.
Similarly, cooperative wolves succeed in hunting, again get to eat and reproduce.
Put differently, the explanation for many if not all adaptations and behaviors of living creatures nature can be “The ones who did not have the features we currently see did not survive”.
The Role of Temperature
Temperature, in addition to moisture, dictates what ecosystem with characteristic vegetation and associated animals (i.e. a biome) exists in a particular place on Earth.
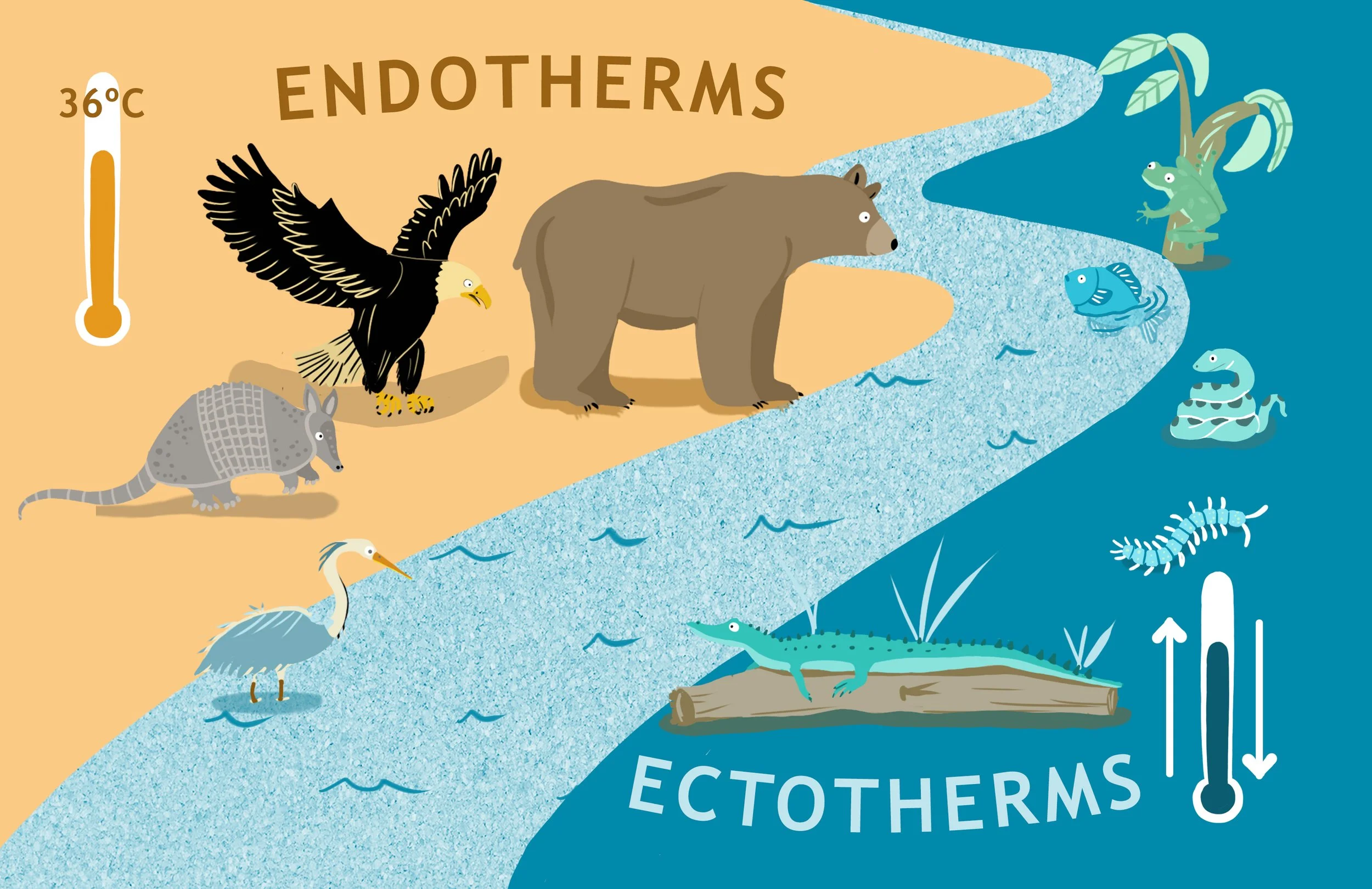
When it comes to the temperature of their environment, animals can be grouped in two main categories.
Fish, reptiles, snakes are ectotherms. Bird and mammals are homeotherms.
· The first is made up of insects, fish, amphibians, snakes, which are called ectotherms, or poikilotherms. They do not regulate their temperature, they simply take the temperature of the environment. Therefore, they have a very narrow optimal range for where they can survive, hence need more constant, or limited temperature variation. That’s why we tend to see more species of reptiles and insects in the tropics.
· The second category is birds and mammals, which are are homeotherms: they tend to keep a constant temperature, and use fur and feathers for insulation from the environment. To maintain their optimal range, they shiver, hibernate, pant etc. As a result, they have a much wider array of temperatures they can live in: insulation and behavior greatly extend the range. See for example the arctic fox or the polar bear, that can be just fine in -25 Fahrenheit and even colder environments.
3. The Matter Cycle
The matter cycle is an important piece of environmental science, and helps in the understanding of pollution and the management of waste and litter. A fundamental law of chemistry and physics, the law of conservation of matter states that matter cannot be created or destroyed (unless there is a nuclear reaction like splitting the atom which releases additional energy), it only changes from one form or another.
In layman’s terms, what this means is that you cannot really throw anything away! There is no “away”, everyhing goes somewhere, is transformed to something else, or cycles through from one form to another.
For example, plants take sunlight and water, and make oxygen and glucose /sugar (for example, the sweetness of fruit or maple syrup).
The chemical compounds change form but do not disappear. Here’s the chemical formula for photosynthesis in plants:
6 CO2 + 6 H20 => (with solar energy) => C6H12O6 + 6 O2
Non-combustible gas + water => high energy sugar or glucose + combustible gas
Animals then take in oxygen, and burn sugar to get energy and create carbon dioxyde (CO2, water, or H2O (i.e. they pee!), and energy.
So we have the same formula, but going the other way, when animals breathe.
Again, the chemical compounds change form, but do not vanish from the face of the Earth. Here’s the chemical formula for respiration in animals:
Chemical components change form and enable life on Earth
6 CO2 + 6 H2O + Energy <= C6H12O6 + 6 O2 (arrow goes the other way)
Non-combustible gas + water + energy <= high energy sugar or glucose + oxygen
When it comes to types of matter cycling through nature, the water cycle is the most familiar biogeochemical cycle, and the most obvious example, since everyone is familiar with rain, evaporation, condensation, to name just a few of the phases in the cycle.
Other key elements cycle through as well, with six of them elements making up 95% of mass of all living organisms: Carbon, Oxygen, Hydrogen, Nitrogen, Phosphorus, Sulfur.
These are called macronutrients because you need lots of them to be a living organism, and they all cycle through nature: the phosphorous cycle, the sulfur cycle etc.
4. Energy
There is a saying that goes “Matter cycles but energy flows”. You can see matter, but cannot see energy.
However, one can see the effects of energy: wind itself is invisible, but its effects are instantly noticeable. Similarly, electricity is invisible, yet again its effects are usually very much in clear view.
Energy can be mechanical, chemical, electrical or nuclear.
It can be kinetic (things are in motion right now) or potential (it is stored, for example in a battery).
Similar to matter, energy cannot be created or destroyed, it only changes from one form to another.
The Fllowing Laws of Matter and Energy are Relevant to the Study of Our Environment
A. The Law of Conservation of Matter: burning garbage creates air pollution and toxic ash
B. The Law of Conservation of Energy: all energy entering a light bulb becomes heat and light
C. Energy Degradation: there is no such thing as perpetual motion (or perpetuum mobilae, as it is called in Latin) without further energy input. Energy flows from high quality to lower quality level; and in any transformation some energy is converted to low quality heat, and is lost fom further use. This is also known as the second law of thermodynamics.
5. Food Chains
Food chains are complicated. Distorting them can have unexpected consequences.
At their simplest, food chains define “who usually eats who & what in order to survive”, and can have 3-4 trophic levels or more, sometimes making complex food webs.
Importantly, at each energy transformation of the food chain, about 10% gets through, 90% gets dissipated as lower quality energy.
For example, from an initial light energy of 100 units, about 10 units pass to corn, then about 1 unit in the turkey who eats the corn, and only about 0.1 units finally to a human being eating the turkey.
As a result of the low efficiency factor/ transmission of energy as it is consumed/ transferred in the food chain, we typically have energy pyramids: few tertiary consumers at top, more secondary consumers below, even more primary consumers underneath, and at bottom many, a lot more producers.
This is the vegetarians’ argument: more energy is available lower down the food chain.
6. Ecology
Ecology is a relatively newer science involving the study of organisms in their environments, sometimes defied as “the study of structure and function of nature”. It is important as it helps us understand what can happen to ecosystems.
For example, the alteration of the biochemical cycle such as through the presence of Phosphorus in water can result in dramatic changes in aquatic life.
Energy can be disrupted through changes in the atmosphere as a result of the accumulation of greenhouse gases. Biomagnification and bioaccumulation are additional potentially adverse effects of pollution, as a result of a gradual concentration of toxic chemicals in living organisms:
Bioaccumulation takes place in a single organism over the span of its life, resulting in a higher concentration in older individuals.
Biomagnification takes place as chemicals transfer from lower trophic levels to higher trophic levels within a food web, resulting in a higher concentration in apex predators, similar to the 10/90% energy transfer but in reverse.
Conclusion
Have I convinced you yet? It is essential for everyone to have a basic understanding of environmental science and its fundamental elements.
By grasping concepts such as the matter cycle, energy transfers, food chains, and ecology, individuals can gain a deeper appreciation for the interconnectedness of the natural world and their role within it:
· Understanding the matter cycle helps us recognize that resources are finite and there is no “away” for our waste. By realizing the importance of reducing, reusing, and recycling, we can contribute to minimizing waste and preserving valuable resources.
· Energy transfers teach us about the delicate balance required to sustain life on Earth. Recognizing the impact of our energy choices can inspire us to adopt renewable sources and reduce our carbon emissions, contributing to a cleaner and healthier planet.
· Learning about food chains highlights the intricate web of relationships between organisms and their environment. It prompts us to reflect on the impact of our dietary choices and the consequences of agricultural practices. By making informed decisions, such as opting for locally sourced and organic food, we can support ecosystems and promote healthier, more sustainable food systems.
· Lastly, understanding ecology allows us to comprehend the complex interactions between organisms and their environment. By appreciating the delicate balance of ecosystems, we become advocates for conservation (using nature wisely and sustainably) and preservation (keeping some ecosystems in their original unaltered condition). We can act to protect biodiversity and mitigate the harmful effects of pollution and climate change.
Ultimately, understanding environmental science empowers us to make informed decisions and become responsible stewards of our world.
By cultivating this understanding, we can collectively work towards a brighter and more harmonious coexistence with Nature, for ourselves and generations to come.
References
1. Udemy.com “Everyone’s World”, by Dr. David Hackett Assistant Professor / Faculty of Arts and Science – Biology and Chemistry, at Nipissing University in Ontario Canada